Vanoxide HC Prescribing Information
Package insert / product label
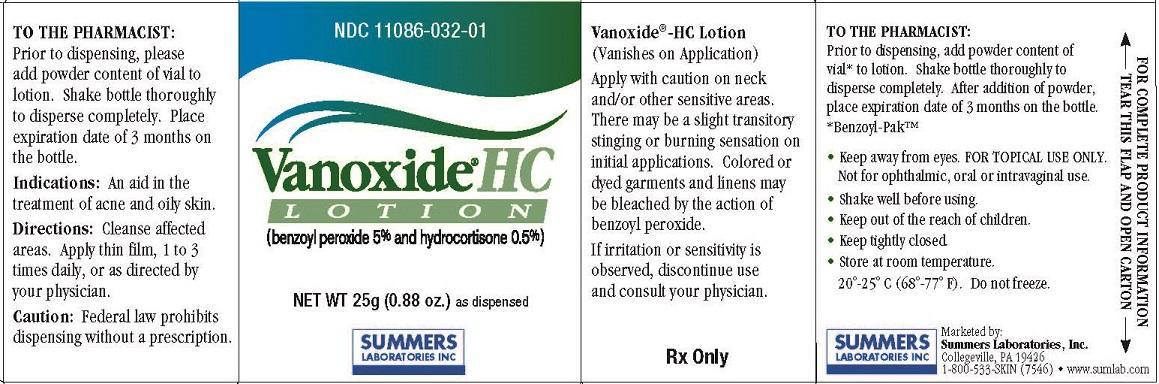
Generic name: benzoyl peroxide, hydrocortisone
Dosage form: lotion
Drug class: Topical acne agents
Medically reviewed by Drugs.com. Last updated on Oct 16, 2023.
On This Page
Apply with caution on neck and/or other sensitive areas. There may be a slight transitory stinging or burning sensation on initial applications. Colored or dyed garments and linens may be bleached by the action of benzoyl peroxide. If irritation or sensitivity is
observed, discontinue use and consult your physician.
TO THE PHARMACIST:
Prior to dispensing, add powder content of vial* to lotion. Shake bottle thoroughly to disperse completely. After addition of powder, place expiration date of 3 months on the bottle.
*Benzoyl-Pak™
| VANOXIDE
HC
benzoyl peroxide, hydrocortisone lotion |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Summers Laboratories Inc (002382612) |
More about Vanoxide-HC (benzoyl peroxide / hydrocortisone topical)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (2)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical acne agents
- En español